Biochemistry It is a laboratory-based discipline that combines biology and chemistry, using chemical knowledge and techniques to understand and solve biological problems. At its core, biochemistry focuses on the structures, functions, and interactions of biological macro molecules—like proteins, nucleic acids, carbohydrates, and lipids—which provide the structure of cells and perform many of the functions associated with life.
The Foundations: The Major Classes of Bio molecules
Life is built on four primary classes of complex, carbon-based molecules.
Proteins
- What they are: Polymers made of amino acids, folded into complex 3D shapes.
Key Functions:
- Enzymes: Catalyze (speed up) nearly all chemical reactions in the cell (e.g., DNA poly merase, lac tase).
- Structure: Provide support (e.g., collagen in skin, keratin in hair).
- Movement: Enable muscle contraction (actin and myosin).
- Transport: Carry substances (e.g., hemoglobin transports oxygen).
- Defense: Antibodies fight off pathogens.
Nucleic Acids (DNA and RNA)
What they are: Polymers made of nucleotides.
Key Functions:
- DNA (Deoxyribonucleic Acid): The hereditary material that stores genetic information. It provides the instructions for building and maintaining an organism.
- RNA (Ribonucleic Acid): Acts as a messenger (mRNA) and helper (tRNA, rRNA) to translate the genetic code in DNA into proteins.
Carbohydrates (Sugars and Starches)
- What they are: Molecules composed of carbon, hydrogen, and oxygen (e.g., glucose, sucrose, starch, cellulose).
Key Functions:
- Energy Source: Provide immediate energy (glucose) and stored energy (glycogen in animals, starch in plants).
- Structure: Provide structural support (e.g., cellulose in plant cell walls, chitin in insect exoskeletons).
- Cell Recognition: Act as “ID tags” on the surface of cells.
Lipids (Fats, Oils, Waxes)
- What they are: A diverse group of hydrophobic (water-fearing) molecules.
Key Functions:
- Energy Storage: Long-term, concentrated energy reserve (triglycerides).
- Cell Membranes: The primary component of all cellular membranes (phospho lipids).
- Signaling: Act as hormones (e.g., steroids like testosterone and estrogen) and messengers.
Key Concepts in Biochemistry
Metabolism
- This refers to the entire set of life-sustaining chemical reactions in organisms. It has two main components:
- Catabolism: The breakdown of molecules to obtain energy (e.g., breaking down glucose in cellular respiration).
- Anabolism: The synthesis of all compounds needed by the cells, using energy (e.g., building proteins from amino acids).
Enzymes and Catalysis
- Enzymes are biological catalysts that lower the activation energy of a reaction, allowing it to proceed rapidly at cellular temperatures. They are highly specific for their substrate (the molecule they act upon).
The Central Dogma of Molecular Biology
This describes the flow of genetic information within a biological system:
- DNA → RNA → Protein
- Replication: DNA copies itself.
- Transcription: DNA is transcribed into mRNA.
- Translation: mRNA is translated by ribosomes into a specific sequence of amino acids, forming a protein.
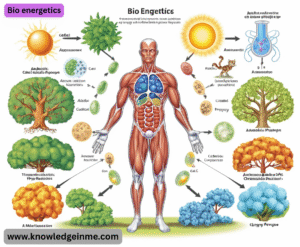
Bio energetics
- This is the study of the transformation of energy in living organisms. A key molecule is ATP (Adenosine Triphosphate), the “energy currency” of the cell. Energy from catabolic reactions is used to create ATP, which then powers anabolic reactions and other cellular work.
Why is Biochemistry Important? (Applications)
Biochemistry is fundamental to many fields:
- Medicine: Understanding diseases at a molecular level (e.g., cancer, diabetes, Alzheimer’s), developing pharmaceuticals, and clinical diagnostics (e.g., blood tests).
- Agriculture: Developing genetically modified crops, improving nutritional content, and creating pesticides.
- Nutrition: Understanding how food fuels the body, the role of vitamins and minerals, and metabolic diseases.
- Biotechnology & Genetic Engineering: Producing insulin, growth hormones, and other therapeutics; CRISPR gene editing.
- Forensics: DNA fingerprinting to identify individuals.
The History and Key Figures
- 18th-19th Century: Early studies on digestion, fermentation, and respiration laid the groundwork.
- 1897: Eduard Buchner discovered that cell-free extracts of yeast could ferment sugar, proving that biochemical processes do not require intact cells. This is often considered the birth of biochemistry.
- 1926: James B. Sumner cry stallized the enzyme urease and showed it was a protein.
- 1953: James Watson, Francis Crick, Rosalind Franklin, and Maurice Wilkins determined the double-helix structure of DNA. This was a revolutionary moment.
- Mid-20th Century: The pathways of central metabolism (e.g., glycolysis, Krebs cycle) were mapped out by Hans Krebs, Carl Cori, Gerty Cori, and others.
Leveling Up: Advanced Biochemical Concepts
The Conformational Dance: Protein Folding and Misfolding
- Proteins don’t just spontaneously form their active shape. The process of protein folding is a delicate dance guided by the sequence of amino acids and assisted by “chaperone” proteins.
- The Problem: Misfolded proteins can aggregate, leading to devastating neurodegenerative diseases known as proteopathies. Examples include:
- Alzheimer’s: Associated with beta-amyloid plaques and tau tangles.
- Parkinson’s: Involves aggregates of a protein called alpha-synuclein.
- Prion Diseases (e.g., Mad Cow): Where a misfolded protein induces other proteins to misfold in a contagious chain reaction.
The Symphony of Signaling: Cell Communication
- Cells don’t operate in isolation. They communicate through complex signaling pathways.
- How it works: A signal (e.g., a hormone, growth factor, or neurotransmitter) binds to a receptor on the cell surface.
- This triggers a signal transduction cascade inside the cell—often a series of proteins activating other proteins through phosphorylation (kinases adding phosphate groups).
- This amplification chain final leads to a cellular response (e.g., gene expression, cell division, or metabolism change).
- Example: The action of insulin is a classic study in biochemistry. It binds to its receptor, triggering a cascade that tells cells to take up glucose from the blood.
Allostery: The Fine-Tuning of Enzymes
- Many enzymes are regulated by molecules binding to a site other than the active site. This is called allosteric regulation.
- An allosteric activator binds and stabilizes the active form of the enzyme, turning it “on.”
- An allosteric inh ibitor binds and stabilizes the inactive form, turning it “off.”
- This is a crucial form of feedback inhibition. For example, the end product of a metabolic pathway often acts as an allosteric inhibitor of an enzyme early in the pathway, preventing the cell from wasting resources.
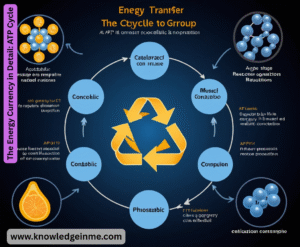
The Energy Currency in Detail: ATP Cycle
ATP is more than just a battery; it’s a constantly cycling system.
- Hydrolysis: ATP + H₂O → ADP + Pᵢ + Energy. This released energy drives endergonic reactions (like muscle contraction or pumping ions).
- Phosphorylation: Often, this energy is used to phosphorylate another molecule (transfer the phosphate group to it), activating it for a reaction.
- Recycling: ADP is then re-phosphorylated to ATP using energy derived from catabolic reactions (like cellular respiration), closing the cycle.
Specialized Sub-Disciplines
- Structural Biology: Focuses on determining the 3D structures of biomolecules.
- Enzymology: The detailed study of enzymes, their kinetics, and mechanisms.
- Meta bolomics: The large-scale study of small molecules, known as metabolites, within cells, biofluids, tissues, or organisms.
- Bioinformatics & Computational Biology: Uses computers to store, analyze, and interpret vast amounts of biological data (e.g., genome sequences, protein structures).
The Biochemical Perspective on a Disease: Cancer
Cancer is fundamentally a biochemical disease. It arises from mutations in genes that code for proteins critical to regulating cell growth and division.
- Oncogenes: Mutated versions of normal genes (proto-oncogenes) that become stuck in the “on” position, promoting uncontrolled growth.
- Tumor Suppressor Genes: Genes whose protein products normally inhibit cell division or trigger cell death (apoptosis). When mutated and inactivated, this crucial brake fails.
- It’s a triumph of structural biochemistry.