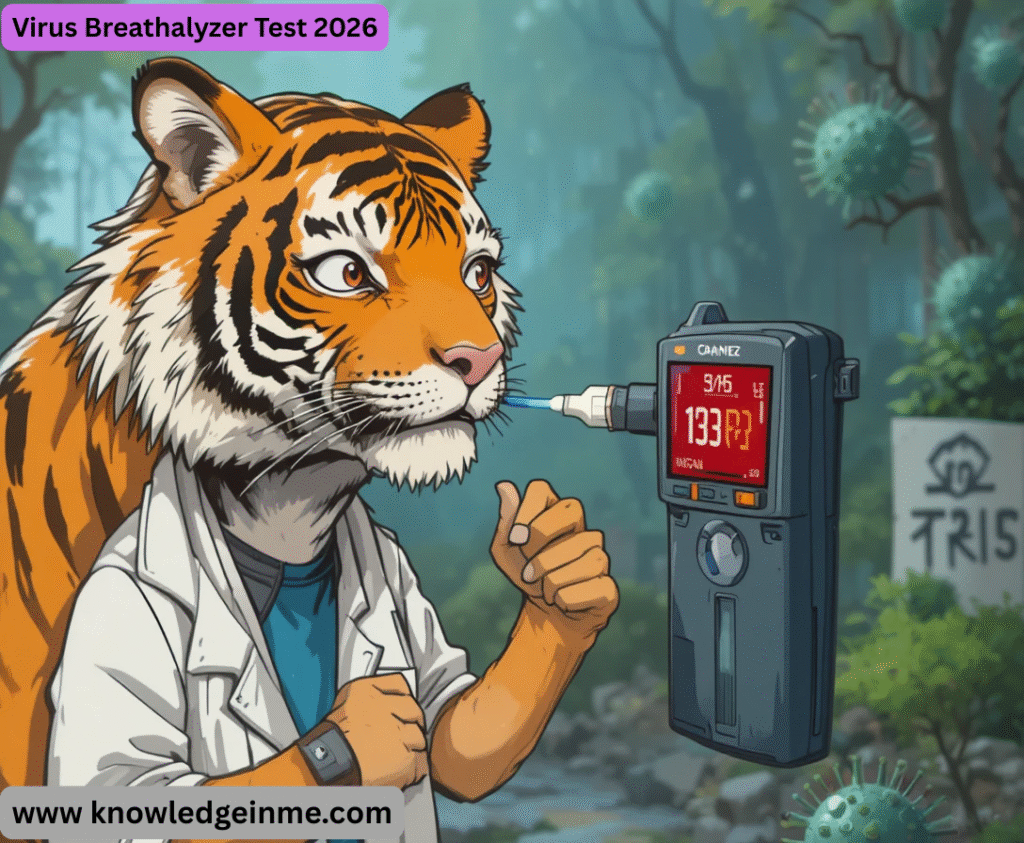
Virus Breathalyzer Test 2026 You’re likely referring to the Tiger BioVirus Breathalyzer, one of the most publicized and advanced devices in this emerging field as of 2026.
Here’s a comprehensive breakdown of the state of virus breathalyzer tests in 2026, focusing on the technology, applications, challenges, and the reality behind the headlines.
The Flagship Example: Tiger BioVirus Breathalyzer
What it is: A rapid, non-invasive diagnostic device designed to detect airborne viral particles (specifically, SARS-CoV-2) in a person’s exhaled breath.
How it (reportedly) works:
- Breath Capture: A person breathes normally into a disposable mouthpiece for about 30-90 seconds.
- Sample Analysis: The exhaled breath condensate and aerosols are analyzed in real-time using a combination of technologies, likely including:
- Mass Spectrometry: Identifying unique molecular fingerprints of viruses.
- AI-Powered Sensors / “Electronic Nose”: Using arrays of nanosensors that react to volatile organic compounds (VOCs) associated with viral infection or the virus itself.
- Result: Delivers a “Positive” or “Negative” result for an active viral infection in under 3 minutes, without needing a lab.
Broader Landscape in 2026
The Tiger system is part of a larger trend. In 2026, breathalyzer-style diagnostics are in a phase of advanced validation and early, targeted deployment.
Key Applications:
- High-Throughput Screening: Airports, stadiums, cruise ships, corporate offices, and hospitals for rapid triage.
- Complement to PCR: Not as a replacement for gold-standard lab tests, but as a supremely fast and convenient first-line screening tool to identify potentially infectious individuals.
- Focus on Respiratory Viruses: Primarily validated for COVID-19, Influenza A/B, and RSV.
- Infection Control: Quickly screening patients and visitors in healthcare settings to prevent outbreaks.
The Science & Technology Behind It
- The core challenge is detecting incredibly low concentrations of specific viral particles in a complex mix of breath components (water vapor, VOCs, gases).
- VOC Profiling: When your body fights a virus, it produces unique metabolic byproducts (VOCs) that you exhale. Breathalyzers can be trained to recognize these “chemical signatures.”
- Direct Particle Capture: Some devices aim to capture and identify viral RNA or antigens directly from breath aerosols, similar to a nasal swab but from the air you exhale.
- AI & Machine Learning: Crucial for interpreting the complex sensor data, distinguishing between different viruses, and reducing false positives from other conditions (e.g., asthma, diet).
Current Challenges & Limitations (As of 2026)
While promising, the technology isn’t a magic wand:
- Regulatory Hurdles: Gaining full FDA/EMA approval as a primary diagnostic tool (vs. a screening tool) requires massive, rigorous clinical trials. Most devices in 2026 operate under Emergency Use Authorizations (EUAs) or are CE-marked for specific use cases.
- Sensitivity vs. PCR: Breath tests are generally less sensitive than lab-based PCR. They excel at detecting high viral loads (when people are most contagious) but might miss early or late-stage infections with low viral shedding.
- Specificity: Can they perfectly distinguish between, say, COVID-19 and a novel rhinovirus? Cross-reactivity remains a challenge.
- Standardization: No universal standard exists for breath collection or analysis, making it hard to compare results across different devices.
Beyond the Headlines: The Deeper Technical & Commercial Battlefield
- The race isn’t just about science; it’s about proving utility, achieving profitability, and navigating a post-pandemic market.
The Two Competing Technological Philosophies:
The “Specific Capture” Camp (e.g., InspectIR, SpiroNose derivatives):
- Goal: Detect the virus itself (viral particles, antigens, or RNA).
- Method: Uses biological components like antibodies or aptamers on a sensor chip to bind specifically to SARS-CoV-2 or influenza proteins captured from breath.
- Pro: High specificity for the target virus. Conceptually similar to a lab test, just with breath.
- Con: More complex, potentially less stable reagents (antibodies can degrade), and may be limited to one virus per test cartridge.
- The “Metabolic Sniffing” Camp (e.g., Owlstone Medical’s VOC fingerprinting):
- Goal: Detect the body’s unique response to infection—the VOC signature.
- Method: Uses gas chromatography and mass spectrometry (GC-MS) or chemical sensor arrays to profile thousands of VOCs. AI then finds the pattern linked to, e.g., COVID-19.
- Pro: Can potentially identify many conditions (infections, cancers, liver disease) with one platform. The “smell” of disease.
- Con: The signature can be confounded by diet, medication, other illnesses (e.g., diabetes). Requires massive, diverse datasets to train the AI reliably.
The “Killer App” Problem:
In 2026, the urgent panic of 2020-2022 has faded. The market is asking:
- “Is this better/faster/cheaper than a rapid antigen test (RAT)?” For home use, the answer is often “not yet.” RATs are $10 and 15 minutes.
- Contagiousness Indicator: Emerging research suggests certain VOC profiles or particle levels correlate better with culturable virus (actual transmission risk) than PCR Ct values or antigen tests. This is a huge selling point for infection control.
- Ultra-Early Detection: Some studies claim VOC shifts occur before symptoms and even before viral load is high. This is still controversial but could be revolutionary for pandemic “nip-in-the-bud” strategies.
The Regulatory & Hurdle Map in 2026:
- EU (CE Mark): Generally the fastest path to market for screening devices. Many breathalyzers are CE-marked as Class I or IIa medical devices.
- USA (FDA): The gold standard and toughest hurdle. In 2026, the FDA is cautiously reviewing these devices. Approval likely requires:
- Dual-claim validation: Proving it works both for diagnosis (in symptomatic) and screening (in asymptomatic).
Comparison to PCR, not just antigen tests.
Extensive data on potential cross-reactivity.
- China (NMPA): A massive market with domestic players (like MGI and BGI) developing their own breath tech. Regulatory pathway is fast-tracked for domestic innovation, especially for public health use.
The Ethical & Privacy “Breathprint” Debate:
- This is a critical, under-discussed aspect. Your breath’s VOC profile is a biometric identifier—a “breathprint”—that reveals profound health information.
- Data Ownership: Who owns the VOC data—you, the device company, the airport, your employer?
- Health Surveillance: Could this enable a new level of workplace or government health monitoring? Breath tests at office entrances could log your respiratory health daily.
- Insurance Implications: Could patterns in breath data be used for risk assessment by health or life insurers?
- Realistic Scenario: A Day in 2026 with Virus Breathalyzers
07:30 AM: A teacher with a scratchy throat visits a local pharmacy clinic. Instead of a nasal swab, she breathes into a kiosk-style breathalyzer. In 2 minutes, it rules out COVID-19 and Influenza A/B but flags a “non-specific respiratory pathogen” signature. She’s advised it’s likely a common cold and given a note for work. - 11:00 AM: A passenger at Frankfurt Airport on a flight from a region with a novel flu outbreak is directed to a rapid screening lane. A breath test clears them in 90 seconds, allowing them to bypass secondary PCR testing and a potential 6-hour quarantine wait.
- 02:00 PM: In an ICU, a ventilator-connected patient is monitored by an in-line breath sensor. It provides continuous, real-time data on viral load decline in response to antiviral drugs, allowing for personalized treatment adjustments.